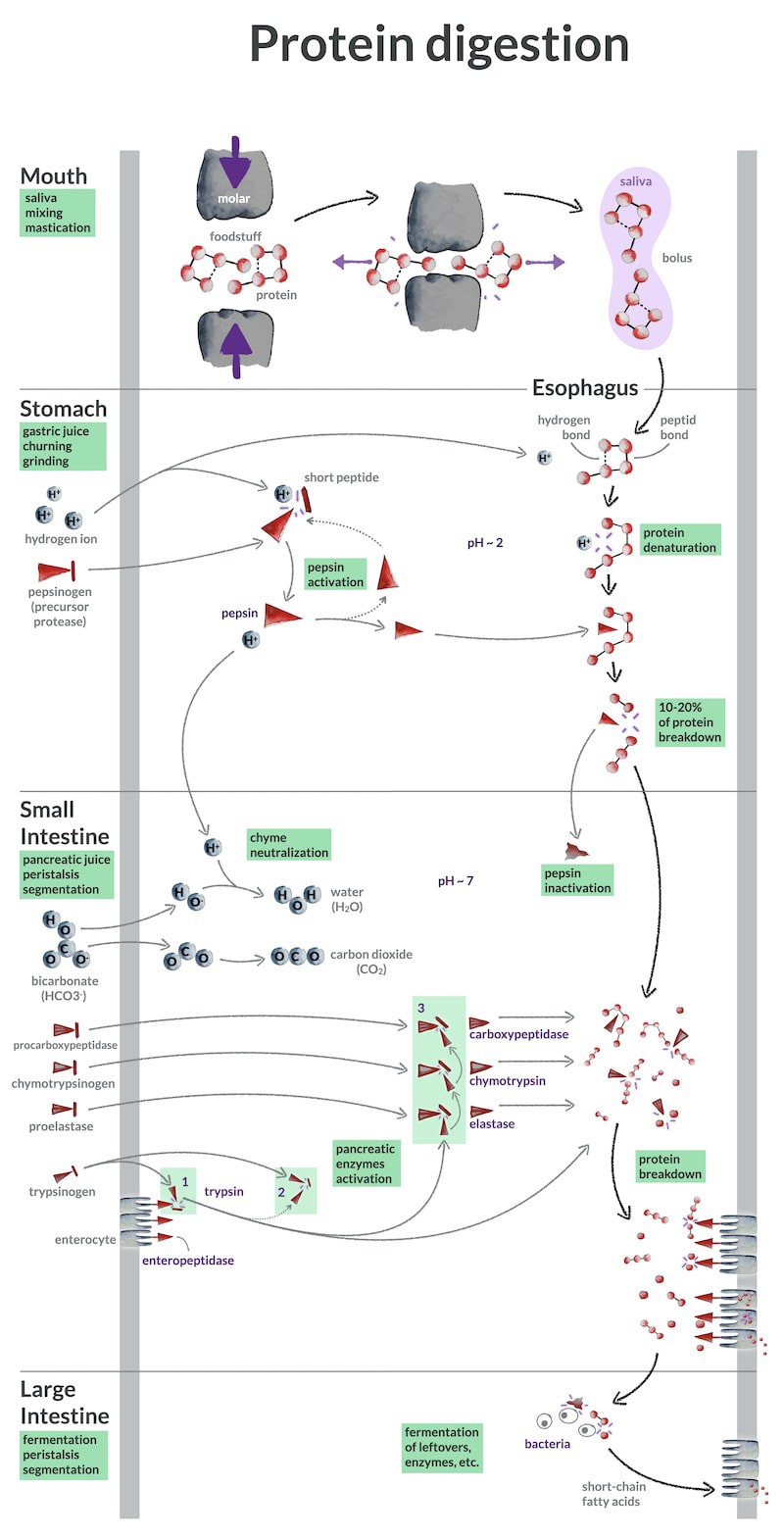
Protein digestion. A visual summary with scientific references
This post is part of my project about the Digestive System.
How does protein digestion occur in the human body? In this post, I integrate visuals with text to explain how proteins are digested along your digestive tract, from mouth to anus.
Proteins are chains of hundreds or thousands of amino acids  linked to one another by strong linkages, called peptide bonds1
linked to one another by strong linkages, called peptide bonds1  . Shorter chains of amino acids are called polypeptides. And, very short chains are called dipeptides
. Shorter chains of amino acids are called polypeptides. And, very short chains are called dipeptides  , tripeptides
, tripeptides  , etc.1.
, etc.1.
Our gut is only able to absorb small molecules: amino acids, di- and tripeptides. Hence, our digestive system needs to find out how to break down all these bonds so that amino acids can be separated from each other.
So how does the process of protein digestion happen, step by step?
Let’s find out by following the fate of a small protein of 5 amino acids 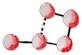 that, say, you are greedily devouring.
that, say, you are greedily devouring.
Table of content
Protein digestion begins in the mouth
As soon as this protein and its nutrients friends enter your mouth, you start to chew them.
You bite with your incisor teeth, you crush with your molars, and you mix food particles and saliva with your tongue. These mastication and mixing, this strong mechanical breakdown, is the first step in protein digestion2.
But this mechanical breakdown does not affect the molecular structure of the proteins.
It does not break the peptide bonds1  , the strong linkages between amino acids
, the strong linkages between amino acids  , that create the chain of amino acids
, that create the chain of amino acids  called the primary structure of the protein.
called the primary structure of the protein.
Nor does it break the weaker bonds, such as hydrogen bonds1  , that maintain the protein chain folded in secondary and even tertiary 3D structures
, that maintain the protein chain folded in secondary and even tertiary 3D structures  .
.
So, if protein structure is not affected by mastication and mixing, why are we saying that protein digestion starts in the mouth?
Because when food fragments are small and coated with saliva 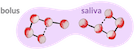 , they are easily moved down by your esophagus, your stomach processes them more efficiently, and their surface area in contact with enzymes is much greater2.
, they are easily moved down by your esophagus, your stomach processes them more efficiently, and their surface area in contact with enzymes is much greater2.
Chewing proteins is thus an important step that prepares for the chemical digestion process later in the stomach and small intestine. In contrast, big chunks of entangled proteins, whose peptide bonds are difficult to reach by enzymes, are more difficult to digest.
Yet, saliva does contain proteases, that is, enzymes that break down proteins. But these proteases, called kallikreins are not directly involved in the digestion process of protein. Instead, they play a role in regulating the blood flow in the salivary glands2.
Eventually, this mechanical action of chewing with your teeth and mixing with your tongue creates a mixture called the bolus, which you propel to the esophagus when swallowing.
Protein digestion in the stomach
But even before you swallow, your stomach is already diligently preparing for the next step.
The walls of the stomach contain small indentations, called gastric pits. Hidden in these pits are cells, the parietal cells2–4, that secrete a digestive juice, the gastric juice.
Among other components, this juice contains hydrogen ions  and chloride ions
and chloride ions  , that is, hydrochloric acid. Yes, you really have hydrochloric acid in your stomach! The release of this very acidic juice by the gastric pits strengthens the acidity in the stomach to about pH 23,5.
, that is, hydrochloric acid. Yes, you really have hydrochloric acid in your stomach! The release of this very acidic juice by the gastric pits strengthens the acidity in the stomach to about pH 23,5.
When the bolus that you propelled into your esophagus lands in the stomach, it discovers a strange cavity whose walls are slowly contracting in waves traveling from top to down. These waves of contraction, called peristalsis, move at a speed of about 1 cm per second2.
At the top of the stomach, the contraction wave has a churning effect. It mixes the bolus with gastric juice, creating a mixture called chyme, where hydrogen ions and enzymes diffuse evenly around proteins2.
Being now soaked into the chyme, under such a strong acidity, our small protein starts to denature, meaning that it begins to lose its 3D structure 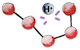 .
This is because acidity disrupts the weak hydrogen bonds
.
This is because acidity disrupts the weak hydrogen bonds  that maintain the protein folded1.
that maintain the protein folded1.
At this stage, however, amino acid chains are still intact, as pH does not break the strong peptide bonds  between amino acids (most of the time)1.
between amino acids (most of the time)1.
But, this unfolding already makes protein much more vulnerable to protease attacks  !
!
Precisely, in the gastric pits, another kind of cell, the chief cells, have also added to the gastric juice a precursor of a protease, pepsinogen  .
While flowing into the stomach, while being mixed with the acidic chyme, pepsinogen undergoes a transformation.
This is because pepsinogen is also a protein, hence its 3D structure is also sensitive to pH.
.
While flowing into the stomach, while being mixed with the acidic chyme, pepsinogen undergoes a transformation.
This is because pepsinogen is also a protein, hence its 3D structure is also sensitive to pH.
In presence of hydrogen ions  , as soon as pH is below 6, pepsinogen
, as soon as pH is below 6, pepsinogen  loses a short series of amino acids
loses a short series of amino acids  and becomes the active protease pepsin
and becomes the active protease pepsin  . Like other proteins, it will end up being digested.
. Like other proteins, it will end up being digested.
Interestingly enough, once pepsin is activated, it also acts as an autocatalyzer, an accelerator, on pepsinogen conversion2,5! Leading to an even faster activation rate of pepsin!
Pepsin starts to break down peptide bonds between amino acids2,6  , creating smaller
, creating smaller  and smaller
and smaller  polypeptides.
polypeptides.
Overall in the digestion process, pepsin accounts for 10-20% of protein digestion2,3.
While the chyme is being mixed at the top of the stomach, while our small protein loses its 3D shapes and even starts to break apart, losing some of its peptide bonds, the wave of contraction reaches the bottom of the stomach.
On its lower side, your stomach is narrower, with thicker muscular walls. Hence, the contraction wave starts to have a stronger and stronger grinding effect. This fragments, even more, the remaining chunks that you didn’t chew completely2.
Eventually, when the wave reaches the bottom of the stomach, a last strong contraction releases a small spurt of chyme into the small intestine while pushing the rest of the chyme backward for further churning and grinding2,3.
By releasing the chyme like this, bit by bit, at a regular pace, the stomach plays a very important role. Even if, craving for food, you have swallowed your meal in just a few minutes, your dutiful stomach accepts everything with compassion, slows down this tsunami of food, hence protecting your next delicate and busy digestive organs so they have enough time to properly process everything.
But, if pepsin only accounts for 10-20% of protein digestion, who is going to complete the job?
Protein digestion in the small intestine
While your stomach is busy secreting, mixing, churning, the pancreas is already diligently preparing for the next step.
In the pancreas, some cells, called acinar cells, secrete 4 main proteolytic enzymes — the pancreatic proteases — into a mixture called the pancreatic juice4.
Like for pepsin in the stomach, these 4 pancreatic proteases are secreted as inactive precursors3,6: trypsinogen  , chymotrypsinogen
, chymotrypsinogen  , proelastase
, proelastase  , and procarboxypeptidase
, and procarboxypeptidase  .
.
As this juice full of enzyme precursors starts to flow through the pancreatic duct towards the intestine, some other cells, the pancreatic duct cells, add into the mixture a high concentration of bicarbonate (HCO3-)3,4  , until the pancreatic juice reaches around pH 7.5-87.
, until the pancreatic juice reaches around pH 7.5-87.
By the time when the chyme, projected by your stomach, pours into the first segment of your small intestine — the duodenum —, with partially digested proteins and polypeptides, the pancreatic juice is ready to enter the scene.
First, this alkaline pancreatic juice quickly neutralizes the acidic chyme, preventing it from damaging the lining of the intestine3.
Then, the pepsin enzyme  becomes inactive. This is because, as we saw in the stomach, pepsin is a protein, thus its shape is sensitive to pH. While alkalinity rises, pepsin activity stops at pH 5, and by the time when pH reaches 6.5 our poor enzyme even starts to denature8
becomes inactive. This is because, as we saw in the stomach, pepsin is a protein, thus its shape is sensitive to pH. While alkalinity rises, pepsin activity stops at pH 5, and by the time when pH reaches 6.5 our poor enzyme even starts to denature8  .
.
In this new alkaline environment, the pancreatic enzymes are about to take over the work of protein digestion. These proteases being much more powerful than pepsin, the greater part of protein digestion occurs here, in the duodenum and upper jejunum3, the first two segments of the small intestine.
But, do you remember, that, at this point, the pancreatic enzymes are still inactive? Their activation is performed through a little complex and quite interesting process! Let’s see how…
As I also described in the article about the area of the digestive tract, the inner intestine wall folds into very small finger-like structures called villi. This multitude of villi on the intestine wall looks like a smooth brush, thence its name — the brush border2.
Then again, these villi are covered with epithelial cells, the enterocytes, whose membrane folds into even smaller finger-like structures called microvilli  .
.
Among many functions, such as the absorption of nutrients, the brush border also plays a role in digestion.
Enterocytes of the duodenum and the jejunum secrete different kinds of proteases — enteropeptidases  — also called enterokinases. These funny enzymes are not liberated in the lumen of the small intestine. Instead, they stay anchored to the microvilli of the enterocytes’ membrane
— also called enterokinases. These funny enzymes are not liberated in the lumen of the small intestine. Instead, they stay anchored to the microvilli of the enterocytes’ membrane  .
As they are hooked to the membrane, they are also referred to as brush border enzymes3.
.
As they are hooked to the membrane, they are also referred to as brush border enzymes3.
And here we are: these funny brush border enzymes are the surprising activators of our pancreatic enzymes!
As soon as pancreatic enzymes land in the duodenum, as soon as they come into contact with the enteropeptidases, they start to activate.
First, enteropeptidases kickstart the activation by converting trypsinogen  into trypsin
into trypsin  by removing a short peptide3,6
by removing a short peptide3,6  .
Second, trypsin itself helps convert trypsinogen into trypsin!6
Third, trypsin converts: chymotrypsinogen
.
Second, trypsin itself helps convert trypsinogen into trypsin!6
Third, trypsin converts: chymotrypsinogen  into chymotrypsin
into chymotrypsin  , proelastase
, proelastase  into elastase
into elastase  , and procarboxypeptidase
, and procarboxypeptidase  into carboxypeptidase3,6
into carboxypeptidase3,6 !
!
Wonderful!
Once activated, trypsin, chymotrypsin, and elastase hurry up to break down remaining proteins and polypeptides from the chyme into even smaller polypeptide chains of 2  to 6
to 6  amino acids3.
amino acids3.
Carboxypeptidase breaks further down some of these small polypeptides into individual amino acids3  .
.
Finally, enteropeptidases, our brush border enzymes, break small peptides down to tripeptides  , dipeptides
, dipeptides  , and amino acids
, and amino acids  3.
3.
But, as well as in the stomach, the digestion process in the small intestine is not just a chemical mechanism. It is also mechanical. While all the chemical reactions happen, the intestine has fun contracting itself in two ways: peristalsis and segmentation.
As in the stomach, some waves of contraction are propelling the chyme along the small intestine2. This is called peristalsis.
Sometimes, the small intestine contracts at different points and maintains its contraction for a while before relaxing and contracting again on adjacent segments2. This is called segmentation.
Peristalsis and segmentation in the small intestine have several functions2. They mix the acidic chyme coming from the stomach with the alkaline pancreatic juice, they ease the mixing of food particles with pancreatic enzymes, and they help the absorption of amino acids.
After this avalanche of activations and breakdowns, most proteins have been digested at this point. Absorption already happens from the duodenum, and continues in the next segments of the small intestine: jejunum and ileum.
Interestingly, enterocytes can absorb not only amino acids but also di- and tripeptides3. Actually, most of the absorption accounts for di- and tripeptides3,6. These peptides are further broken down to amino acids, inside the enterocytes, before being released into the blood3,6.
Protein fermentation in the large intestine
Despite the thorough process of protein digestion that started in the mouth, continued in the stomach, and was mostly done in the small intestine, some proteins still reach the large intestine not totally digested3,4.
These proteins can come from the diet, like elastin and collagen; or from the digestive tract itself, like sloughed epithelial cells, dead bacteria, or pancreatic enzymes, which are also proteins4.
After arriving in the large intestine, these proteins are further decomposed by colonic bacteria, mainly in the distal colon4. This degradation leads to the formation of useful molecules for our body, such as short-chain and branched-chain fatty acids.
Colonic epithelial cells absorb fatty acids4. In the body, they are used as nutrients, and some of them are locally used as an energy source by epithelial cells of the colon.
Wrap up
Proteins are chains of amino acids folded into 3D structures  . Protein structure is maintained thanks to different kinds of bonds, mainly peptide
. Protein structure is maintained thanks to different kinds of bonds, mainly peptide  , and hydrogen bonds
, and hydrogen bonds  . The digestion process breaks down these linkages, converting large proteins into free amino acids
. The digestion process breaks down these linkages, converting large proteins into free amino acids  .
.
Although some proteases are secreted in the saliva, they don’t act in protein digestion. Yet, digestion starts in the mouth, with a strong mechanical action of chewing and mixing. Well-chewed food significantly facilitates the chemical digestion of proteins in the stomach.
By combining very low pH, the protein enzyme pepsin, and churning, the stomach efficiently starts the chemical process of protein digestion. Yet, only 10-20% of protein digestion occurs in the stomach itself, leaving most of the protein breakdown work to the small intestine…
Most of the protein digestion process happens at the beginning of the small intestine, in the duodenum, and in the upper jejunum. Proteins partially digested by the stomach are further broken down by several powerful pancreatic proteases and by enzymes hooked on the membrane of the intestinal epithelial cells. The protein digestion process finishes inside these epithelial cells.
Some proteins from the diet or from the digestive tract reach the large intestine undigested. There, they are mainly transformed into short-chain fatty acids by colonic bacteria fermentation, and absorbed by the epithelial cells.
Discussion on science, digestion, and health
This article focuses on the main mechanisms of normal protein digestion, as they are described in scientific textbooks. You can find more detailed information in the textbooks themselves.
I also think it is worthwhile adding a few more thoughts on protein digestion and health, to get a bigger picture.
Antinutrients
Some foods can disrupt this ideal digestion process. For instance, some sources of plant protein, like legumes, contain antinutrients, such as trypsin inhibitors. As you saw, trypsin is a crucial protein enzyme in the small intestine, so its inhibition can significantly impact protein digestion. Soaking, cooking, and fermenting legumes can reduce antinutrient content, hence increasing their digestibility. As an example, I studied in another article how trypsin inhibitors in chickpeas can be reduced by ~90% while making chickpea tempeh.
And also: nutrients absorption, conversion, delivery…
When we try to improve our health with diet, we easily tend to focus on the daily amounts of protein or other nutrient intake. Yet, once in our belly, food still needs to be digested properly, nutrients need to be absorbed through the intestinal wall, converted and stored properly in the liver, and delivered efficiently inside the right cells at the right time. All these stages can potentially be disrupted and contribute to the development of health problems. So, even if the process of protein digestion and other nutrients is very interesting and important, if we want to understand nutrition and make appropriate choices for health, we need to dig deeper.
Scientific knowledge is likely to be very limited
When we see the visual summary above, just about protein digestion alone, with so many arrows and enzymes everywhere, it looks so complex! So, we easily tend, unconsciously, to think that this scientific information is already comprehensive and accurate. However, the more we dig, the more we realize that there are likely to be many errors, unknown enzymes, unknown mechanisms, etc. Especially for very complex topics such as nutrition, health, agronomy, or ecology.
As a simple example, when I researched the length of our digestion tract, I noticed that a textbook published in 2018 was still indicating a length twice higher for the small intestine than the most recent estimate published four years earlier in 2014. Sometimes, even clever researchers and professors forget to check the most basic information, thinking that, as everyone has been repeating them many times for decades, they must be accurate.
Very often, if we try to look for the origin behind some common nutrition or health advice provided by conventional or alternative medicine, we realize that they are based on hypotheses and theories that haven’t really been proven. Yet, we spontaneously think that, if it is advised, it must have been proven and verified many times.
I think scientific knowledge can be very interesting and useful, and I have fun diving into it. But the more I dive, the more I notice that Western societies tend to see in scientific knowledge much more certainty than it is actually able to offer! Knowing this is already being much more armed to face the complexity of the world.
Good resources
If you want to dive deeper into the metabolic pathways behind gastric and pancreatic juices secretion and protein digestion, I recommend having a look at the
Kyoto Encyclopedia of Genes and Genomes .
It proposes nice pathway map along with other detailed information when you click on the different molecules.
.
It proposes nice pathway map along with other detailed information when you click on the different molecules.
Did you enjoy this post?
Great! Then, you may also like to read about the length or the inner surface area of our tract.
Open license
The text, images, and visual summary are under an open license, meaning that you are free to share adaptations, even for commercial purposes! As long as you give credit to my work by mentioning “Elegant Experiments” with a link to www.elegantexperiments.net